Interacción entre un fragmento peptídico del receptor tipo 1 de angiotensina humano y sistemas miméticos de membrana biológica
DOI:
https://doi.org/10.33017/RevECIPeru2015.0019/Keywords:
Human AT1 receptor, circular dichroism, fluorescence, model membraneAbstract
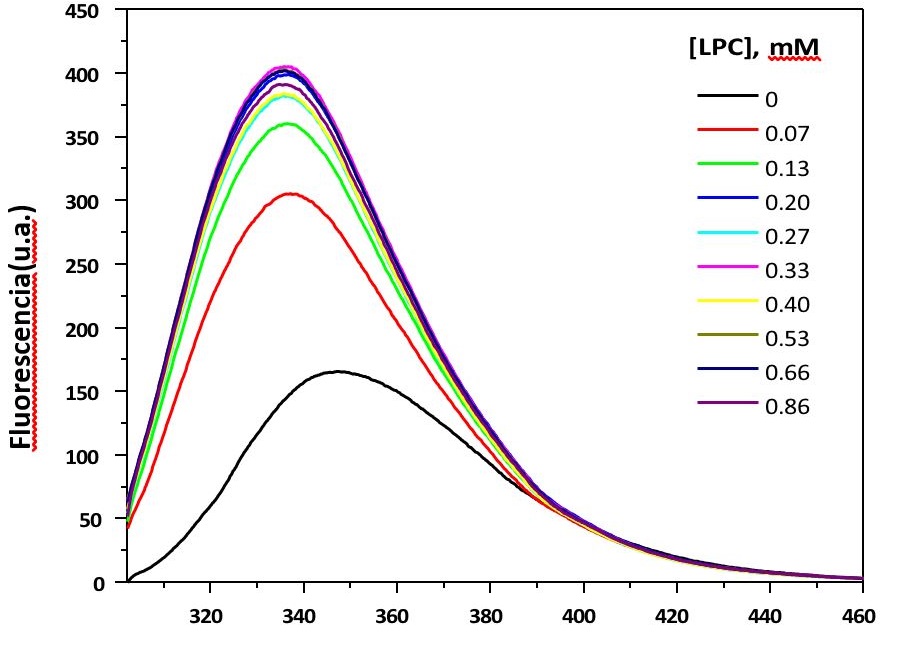
The type 1 angiotensin (AT1) receptor belongs to G protein coupled receptors (GPCR) superfamily. Conformational studies of GPCR peptide fragments require the use of systems that mimic the receptor environment. The current study aims to investigate the interaction of the peptide fragment Ac-YRWPFGNYLCONH2 (fEC1), corresponding to amino acid residues 92 -100 of the first extracellular loop of the human AT1 receptor, with model membranes –micelles and large unilamellar vesicles (LUV). The micelles were prepared from lysophospholipids: 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC) and 1-palmitoyl-2-hydroxysn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) (LPG) and, LUVs were prepared from 1-palmitoyl-2oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) (POPG). The peptide fEC1 was synthesized by solid-phase synthesis. Fluorescence measurements were performed using the tryptophan residue (W94), present in the amino acid sequence of fEC1, as an intrinsec fluorescent probe. The excitation wavelength (exc) was 195 nm. The fluorescence spectra of fEC1 (10 µM) were obtained at increasing concentrations of lysophospholipids or phospholipids. The fluorescence intensity of peptide in the presence of zwitterionic (LPC) and anionic (LPC: LPG, mol:mol) micelles and in the presence of anionic LUV (POPC:POPG, mol:mol) increased. Besides, upon addition of these model systems, a blue-shift of the maximum emission peak of the peptide was observed. The spectral changes observed are due to the sensitivity of tryptophan to the polarity of the medium. From the binding isotherms, binding constants (Kb) were determined. According to these values, the binding of the peptide with the anionic systems was higher than with the zwitterionic ones, this is due to the contribution of electrostatic forces. For fluorescence quenching studies, acrylamide was used as a collisional quencher, thus, SternVolmer constants were determined. According to these data, the fluorophore is more exposed to acrylamide when fEC1 is in solution than in the presence of the biomimetic membrane. Moreover, the values of fluorescence anisotropy (r) which are related to the rotational diffusion of a fluorophore were calculated. According to these data, the peptide showed higher rotational diffusion in aqueous medium than in presence of model systems. The results obtained by steady-state fluorescence show the binding of the peptide fragment fEC1 with lysophospholipid micelles, POPC: POPG LUV and, in lesser extent, with POPC LUV.