Análisis conformacional de un fragmento peptídico del primer bucle extracelular del receptor tipo 1 de angiotensina humano
DOI:
https://doi.org/10.33017/RevECIPeru2017.0009/Keywords:
Human AT1 receptor, circular dichroism, micelles, large unilamellar vesiclesAbstract
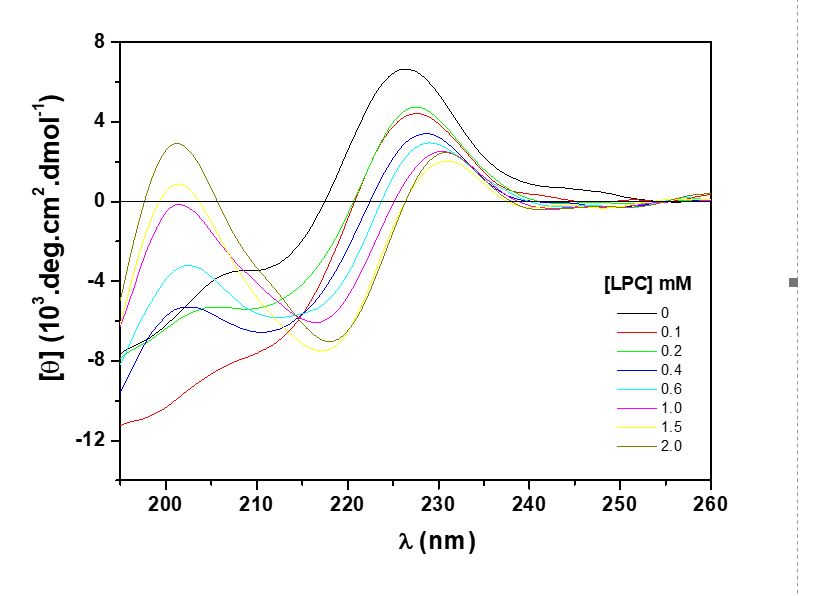
The type 1 receptor for angiotensin (AT1) is an important mediator of the vasoconstrictor functions of angiotensin II. During the activation process of the receiver, it undergoes structural alteration that leads to the process of signal transduction. In the present study, the conformational properties of the peptide fragment Ac-YRWPFGNYL-CONH2 (fEC1) corresponding to the first extracellular loop of the human AT1 receptor both in aqueous medium and in the presence of systems that mimics the biological membrane were investigated. In this way, micelles and large unilamellar vesicles (LUV) -both anionic and zwitterionic in nature- were used as biomimetic systems. The micelles were prepared from the lysophospholipids: 1-palmitoyl-2-hydroxy-phosphatidylcholine (LPC) and 1-palmitoyl-2-hydroxy-phosphatidylglycerol (LPG), whereas, the LUV were prepared from 1-palmitoyl- 1-oleoyl-phosphatidylcholine (POPC) and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG). The peptide was synthesized by the solid phase synthesis method and the conformational analyzes were performed by circular dichroism. The experiments were performed at pH 7.0. At this pH the theoretical charge of fEC1 is +1. The circular dichroism spectrum of fEC1 in solution showed a positive band at approximately 226 nm, whereas in the presence of zwitterionic and anionic micelles and, in the presence of anionic LUV, spectral alterations were observed, that is, the acquisition of secondary structure of the peptide as a consequence of the peptide-biomimetic system interaction.